For One Manufacturer, Vaccination Is a Personal Cause
 At Gilster-Mary Lee Corporation—a food manufacturer headquartered in Chester, Illinois—the impact of COVID-19 is deeply personal. In April 2020, their CEO, Don Welge, passed away due to the virus.
At Gilster-Mary Lee Corporation—a food manufacturer headquartered in Chester, Illinois—the impact of COVID-19 is deeply personal. In April 2020, their CEO, Don Welge, passed away due to the virus.
“It was a time when there was not a lot known about the virus,” said CEO Tom Welge, who is Don’s son. “He was very much the spirit of the company, and we found ourselves without him at a time when demand was blowing up for groceries as everybody began staying home and the supply chain was starting to be disrupted. It was a very challenging year, and we were without our captain.”
A year later, that heartbreaking experience has made Welge especially supportive of the nationwide vaccination campaign and motivated to get his company’s workers vaccinated. He spoke to us recently about Gilster-Mary Lee’s methods for overcoming vaccine hesitancy and its efforts to run its own vaccine clinics.
Reducing vaccine hesitancy: The company is taking a multistep approach to help employees become comfortable with vaccination—from disseminating the NAM’s materials and fact sheets to coordinating with state and local health associations to creating its own informational products. But the most critical piece, according to Welge, is communication.
- “Probably the most important thing is consistent messaging and conversations,” said Welge. “We engaged our managers to make sure they were on board, and then we asked them to go out and evangelize the teams that they work with.”
Open engagement: “You’ve got to be open to answering questions that people have about the vaccine, and not belittle any questions that are brought to you,” said Welge. “At the end of the day, it’s still a decision that an individual has to make—and all we can do is point out all the advantages.”
Vaccination stations: Gilster-Mary Lee isn’t only encouraging its employees to receive the vaccine; the company is also bringing the vaccine directly to them by setting up vaccination clinics at its facilities—a process that was no small feat.
- “You make a lot of calls—you find the right person to talk to at a health care agency or a pharmacy and have a friendly conversation,” said Welge. “We are all aligned on what the mission is: we want to get as many doses to as many people as possible. If you show that you are somebody who will do whatever it takes to make this work, they’ll say let’s work with these guys.”
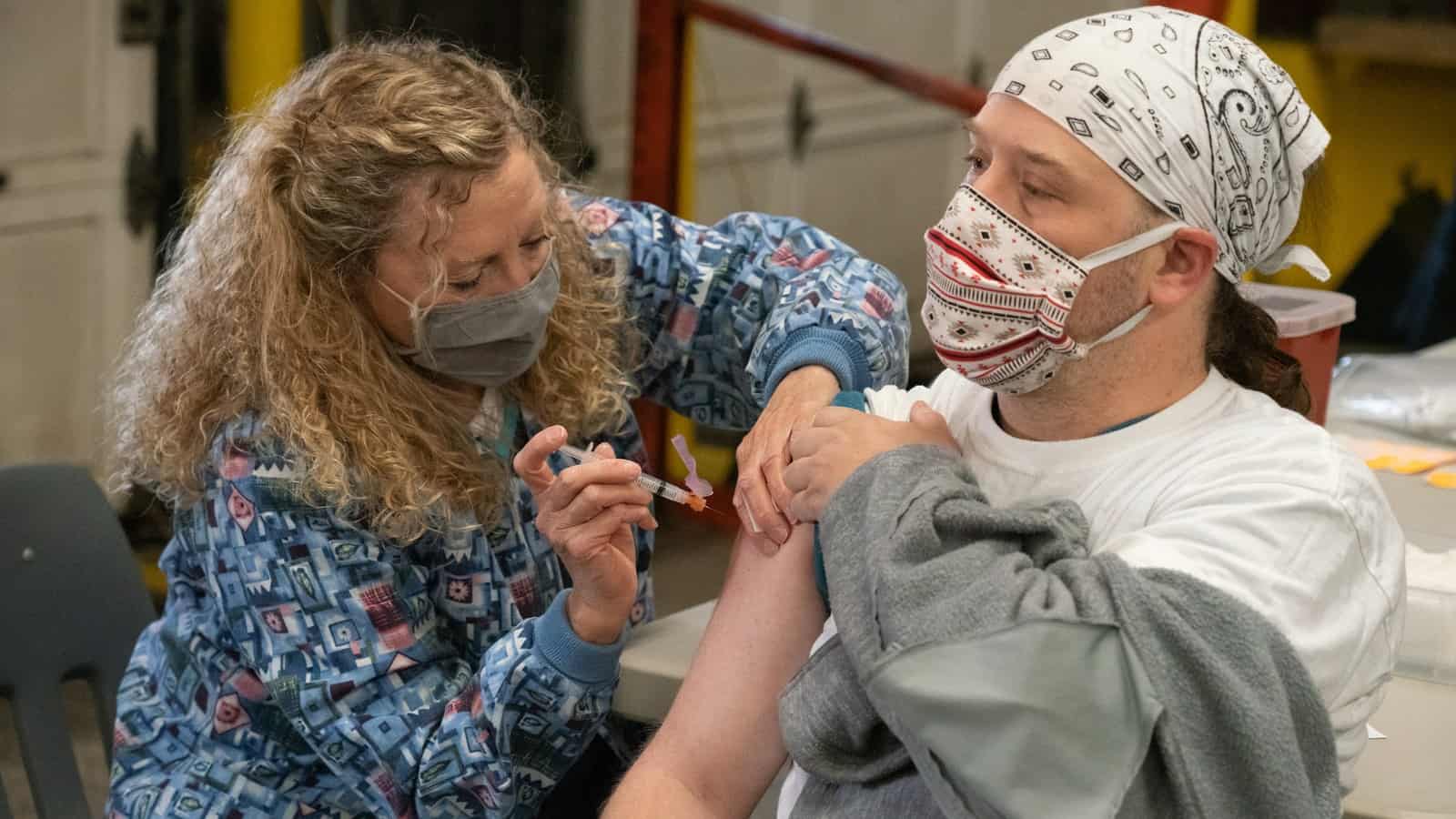
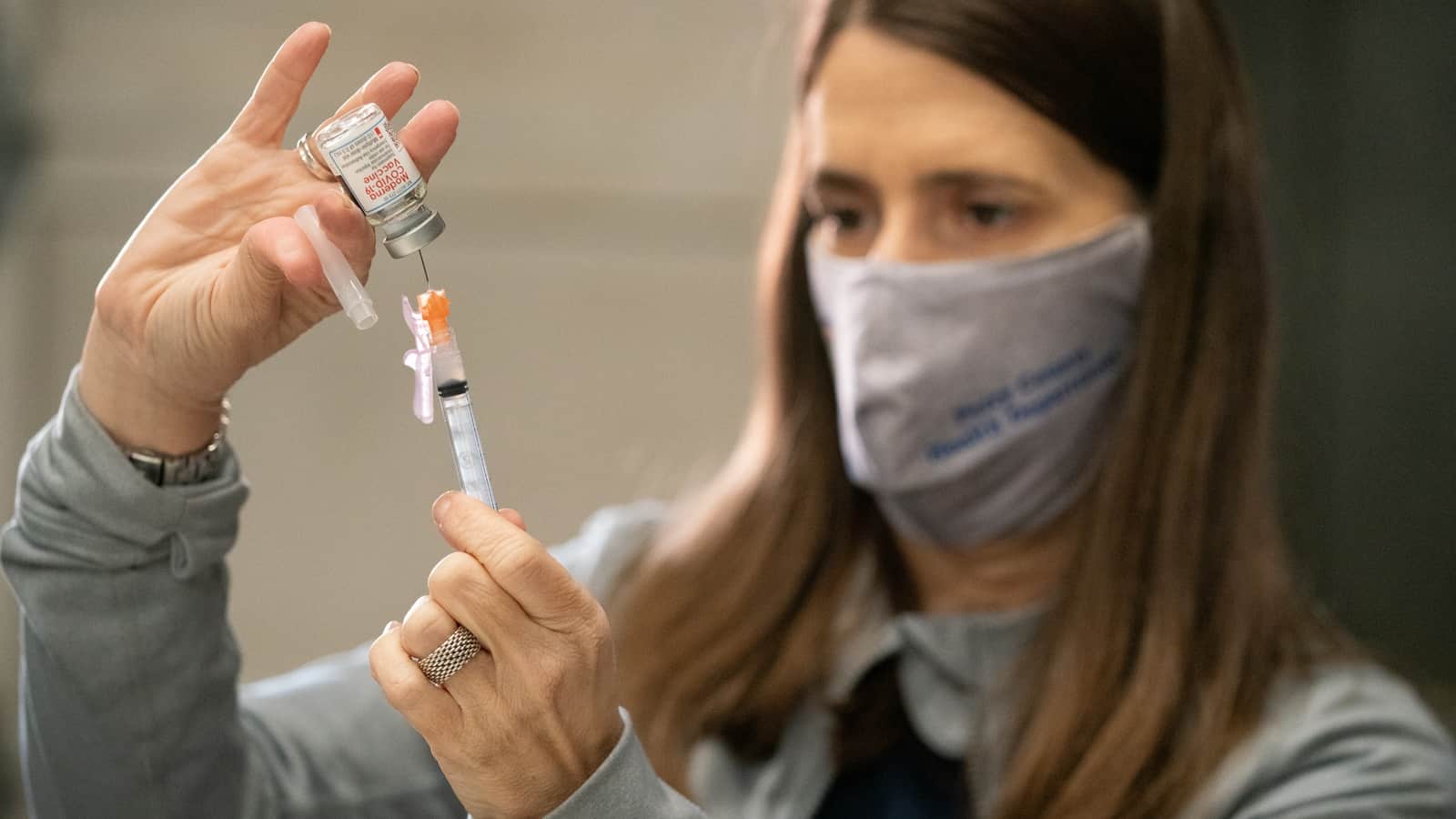
Pictures at a vaccination: NAM Director of Photography David Bohrer captured one of Gilster-Mary Lee’s vaccination events on April 1. The county health department sent over staff to give the Moderna vaccine to more than 150 workers at the company’s Perryville, Missouri, facility.
Here is Perry County Registered Nurse Amy Hector filling a shot from a vaccine vial:
Workers who are coming off an overnight shift or starting their day shift get vaccinated:

A nurse wears a pro-vaccine shirt in the picture below—sending the right message!

And last, getting your first vaccine dose is definitely worth smiling about. Here’s Gilster-Mary Lee employee Claudia Bohnert showing off a new Band-Aid where she received her first shot.

The bottom line: Welge is adamant in his support of vaccinations. As he puts it—and tells his employees—“This is a decision that protects you, protects your family and protects your coworkers.”
Timmons says: NAM President and CEO Jay Timmons said about Welge’s efforts, “Having lost my father to COVID-19, I know what the Welge family has endured. And I know how it strengthens your resolve to see everyone get vaccinated. No one should have to feel the immense pain of losing a loved one to COVID-19. And thankfully, now with the vaccines, we can protect all of our loved ones.”
How Manufacturers Put Together 17 Vaccination Events
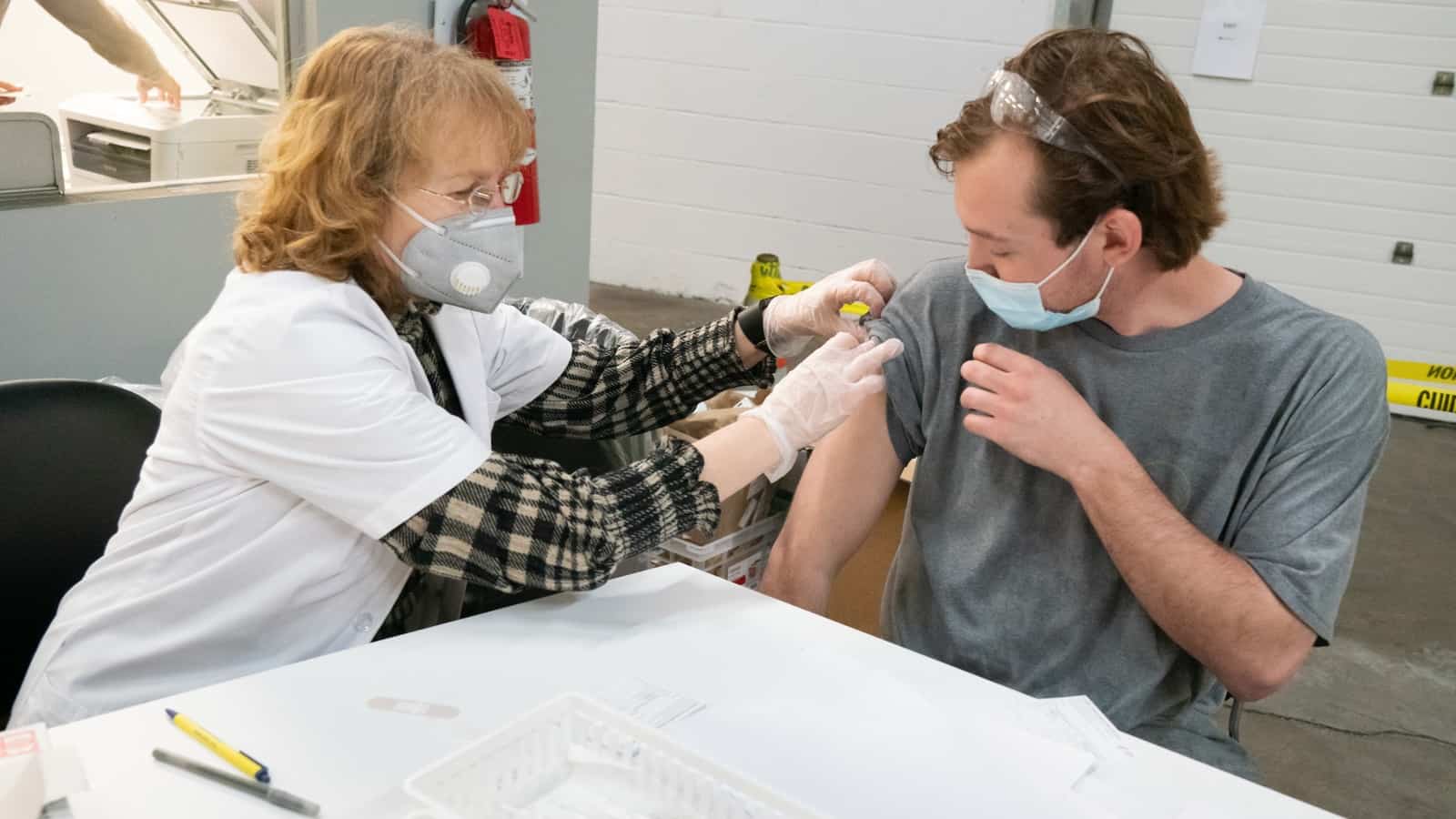
You could say this was just another supply chain challenge for Marlin Steel Wire Products President and Owner Drew Greenblatt—a matter of getting the right materials to the right people at the right time. Except in this case, the right materials were COVID-19 vaccines, the right people were more than 3,300 manufacturing workers at 81 companies, and the right time was ASAP.
Greenblatt organized a coalition of Maryland manufacturers interested in hosting their own vaccination events, which required liaising with government officials and partnering with local pharmacies. The companies will host 17 events at their facilities, bringing in pharmacy employees to administer Pfizer vaccines.
First time: This grand plan went into motion last week, with the first vaccination events held on March 31. More than 120 essential manufacturing employees from Marlin Steel (a wire products and metal fabrication firm), Orlando Products (a disposable and reusable packaging maker) and Arnold Packaging (a producer of packaging and containers) got their shots at the Orlando facility. Meanwhile, a second event took place at the facility of spice manufacturer McCormick for that company’s employees.
NAM Director of Photography David Bohrer attended the Orlando event, capturing the vaccinations in progress. Here is a pharmacy worker explaining the COVID-19 shot record card to an employee who just got his first dose:

And here’s Hector Carmona of Marlin Steel receiving his shot:
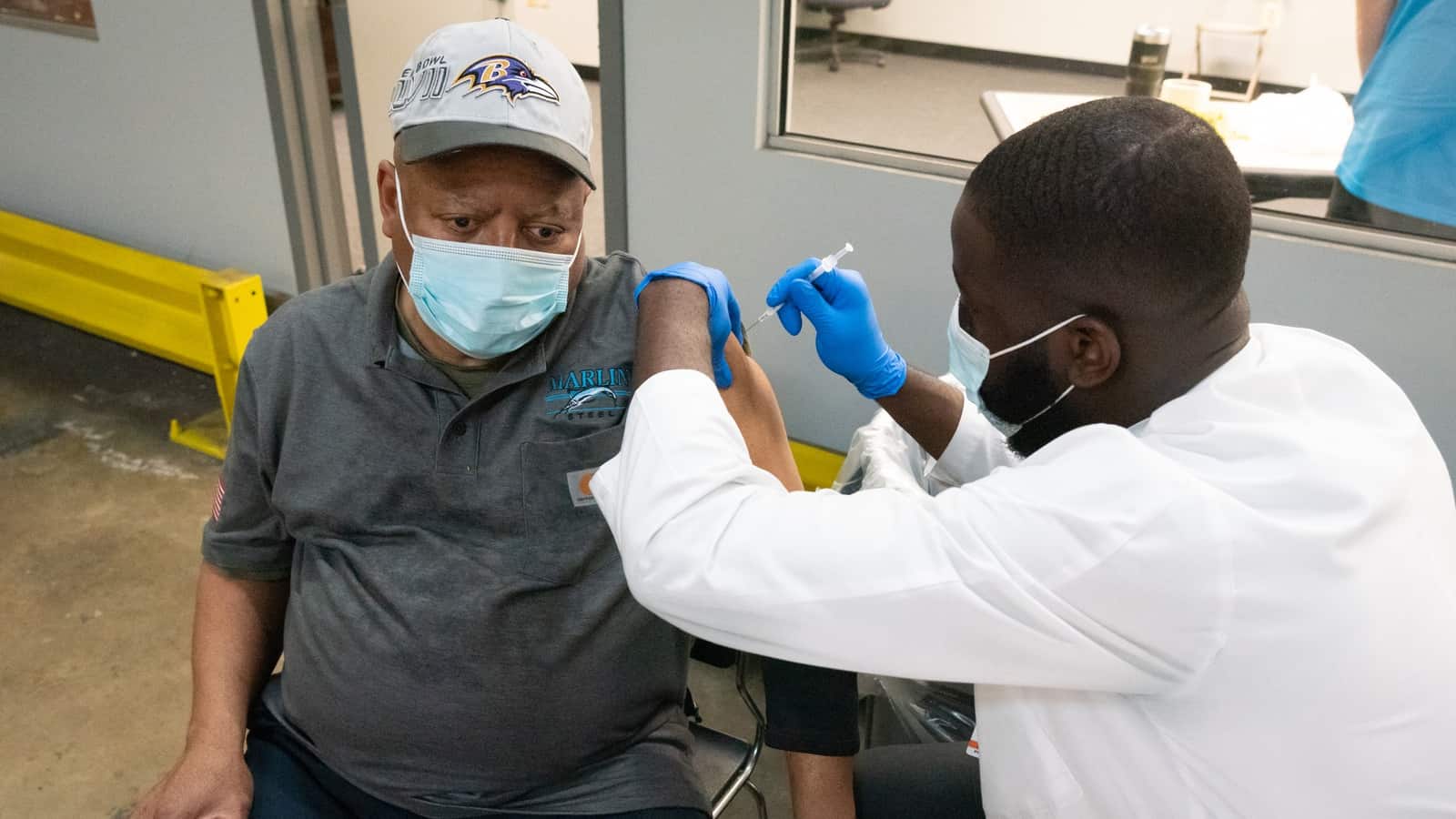
Behind Marlin Steel employee Jake Dieter, you can see other employees waiting the required 15 minutes after they receive their vaccinations (in case of adverse reactions).
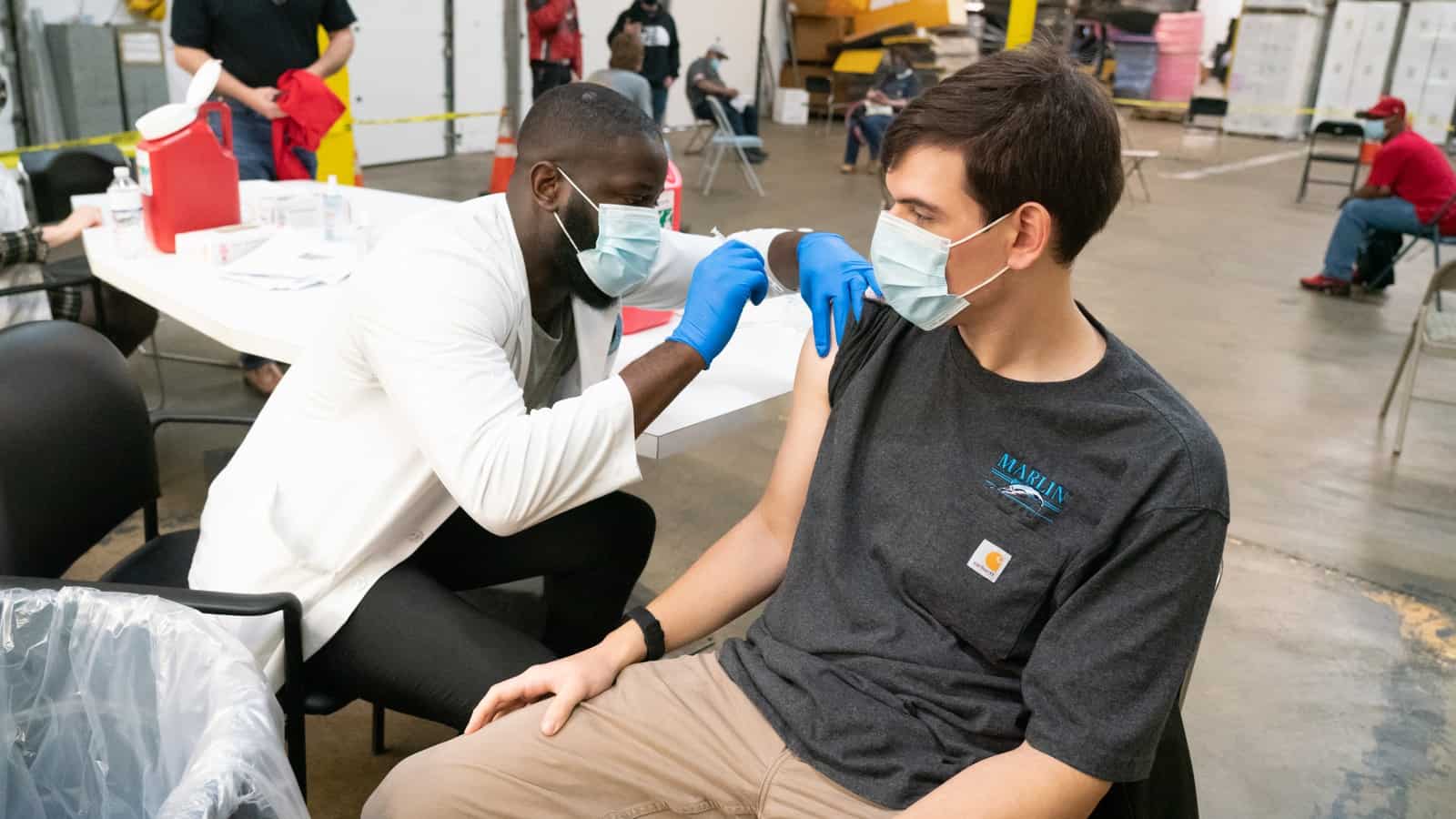
And last, here’s a McCormick employee celebrating her vaccination with a pharmacy employee (this photo was taken by McCormick staff at the company’s event).

How they did it: To help other manufacturers who might be interested in hosting vaccination clinics, Greenblatt explained to us how he planned the events.
The entire process took just under two months, beginning in late January. Greenblatt sprang into action once essential manufacturing workers became eligible for vaccinations in Maryland. Seeing his employees struggle to get appointments, he decided to work with other companies to make things easier for manufacturing workers. This is how they did it:
- First, a project manager at each company assembled a list of “critical” employees who wished to be vaccinated.
- Out of 8,000 employees, they built a spreadsheet of 3,300 essential manufacturing workers who were not vaccinated and were willing to receive the shots.
- At the request of the Maryland governor’s office, Greenblatt’s coalition partnered with Giant, Safeway and Rite Aid to get the vaccine. He jumped at the opportunity to work with efficient private-sector partners, as opposed to a patchwork of county governments. This allowed the coalition to move much more quickly—and to nail down the supply of the vaccine much faster.
- Once all the pharmacies and companies were committed, everything moved very quickly. The companies were warned that they had to be ready to vaccinate “at the drop of a hat,” so once the vaccination dates were confirmed, they coordinated with each other and the pharmacies directly to get workers where they needed to go.
The coalition has had the enthusiastic support of the Maryland government throughout its efforts. Gov. Larry Hogan, whose office was instrumental in arranging the vaccination events, will attend one of them in person in the coming days.
The last word: “We got it done. A mass inoculation blitz of 3,300 essential manufacturing workers in Maryland are getting the vaccine in 17 locations over the next couple days,” said Greenblatt. “Governor Hogan led the charge to make sure our food processing, medical products manufacturing and defense workers are protected against COVID-19. This leadership will keep our nation safe.”
How 5G Is Transforming Manufacturing

More than half of all manufacturers will be testing or using fifth-generation cellular wireless technology (aka 5G) in some capacity by the end of 2021, according to a new study from The Manufacturing Institute. The big numbers:
- 91% of manufacturers believe 5G connectivity will be important to the overall future of their businesses.
- 91% of manufacturers indicate speed of 5G deployment will have a positive impact on their ability to compete globally.
- 88% of manufacturers indicate 5G connectivity will allow engineers to troubleshoot remotely.
All-encompassing: 5G is poised to help manufacturers in almost every part of their businesses, according to the study.
- Nine in ten manufacturers expect the utilization of 5G to lead to the creation of new processes (88%) and new businesses (86%). It can make supply chains more efficient and both machines and workers more productive. It also will likely lead to new improvements no one has anticipated yet.
Drilling down: Let’s look at just one facet of 5G’s potential impact: its effects on factory operations. This is how comprehensive the 5G transformation is expected to be:
- Four-fifths of manufacturers indicate 5G technology will be important to inventory tracking (83%), facility security (81%) and warehousing and logistics (81%) within their facilities.
- Three-fourths of manufacturers indicate 5G will also be important to inspection (76%) and assembly (76%) activities, with seven in ten saying packaging (72%) and employee training (71%) efforts will benefit from the deployment of 5G.
And let’s not overlook the fact that more than 90% of manufacturers expect cost savings of approximately 38% from their 5G connections.
Competitive advantage: “Manufacturers’ competitiveness depends on their ability to continuously improve the efficiency and effectiveness of their operations, and disruptive technologies are changing the way that firms innovate and produce,” said the Institute’s Center for Manufacturing Research Director and NAM Chief Economist Chad Moutray.
More information: You can join the Institute for a webinar on 5G technologies on Tuesday, April 6, at 2:00 p.m. ET. Register here.
NAM and MI Launch Yellow and Red Ribbon Initiative

The NAM and The Manufacturing Institute are launching the Yellow and Red Ribbon Initiative today to encourage vaccinations against COVID-19. Here’s what you need to know to get involved.
What it means: The ribbon is designed with two colors: yellow, which shows that we support one another, and red, which signifies that we care for one another. Wearing or displaying yellow and red ribbons is intended to serve as a sign of support for COVID-19 vaccination and a signal that you have been personally vaccinated.
Why do it: “It’s the same philosophy behind ‘I Voted’ stickers,” said NAM President and CEO Jay Timmons. “Seeing that others have been safely vaccinated sends a powerful message to those who have not yet made the decision to get their shot.”
What you can do: You can wear the ribbon to work or out in public, or in your social media profile pictures. If you’re an employer or run a vaccination site, you can make the ribbons available to those who get their shot.
How to get your pins: The NAM and the MI are producing yellow and red pins to distribute to workers, families and communities and have developed tools such as bilingual social media, email and newsletter copy and posters to support distribution.
- Bulk orders can be made online here. The NAM and MI are also encouraging a grassroots effort in communities nationwide, urging people to make ribbons at home, find them online or locate them at a craft store.
What we’re building on: Getting manufacturers and Americans their COVID-19 vaccines is a key step toward ending the pandemic. See how the NAM and MI are working to get that done through its “This Is Our Shot” project.
What It Takes to Manufacture a Vaccine
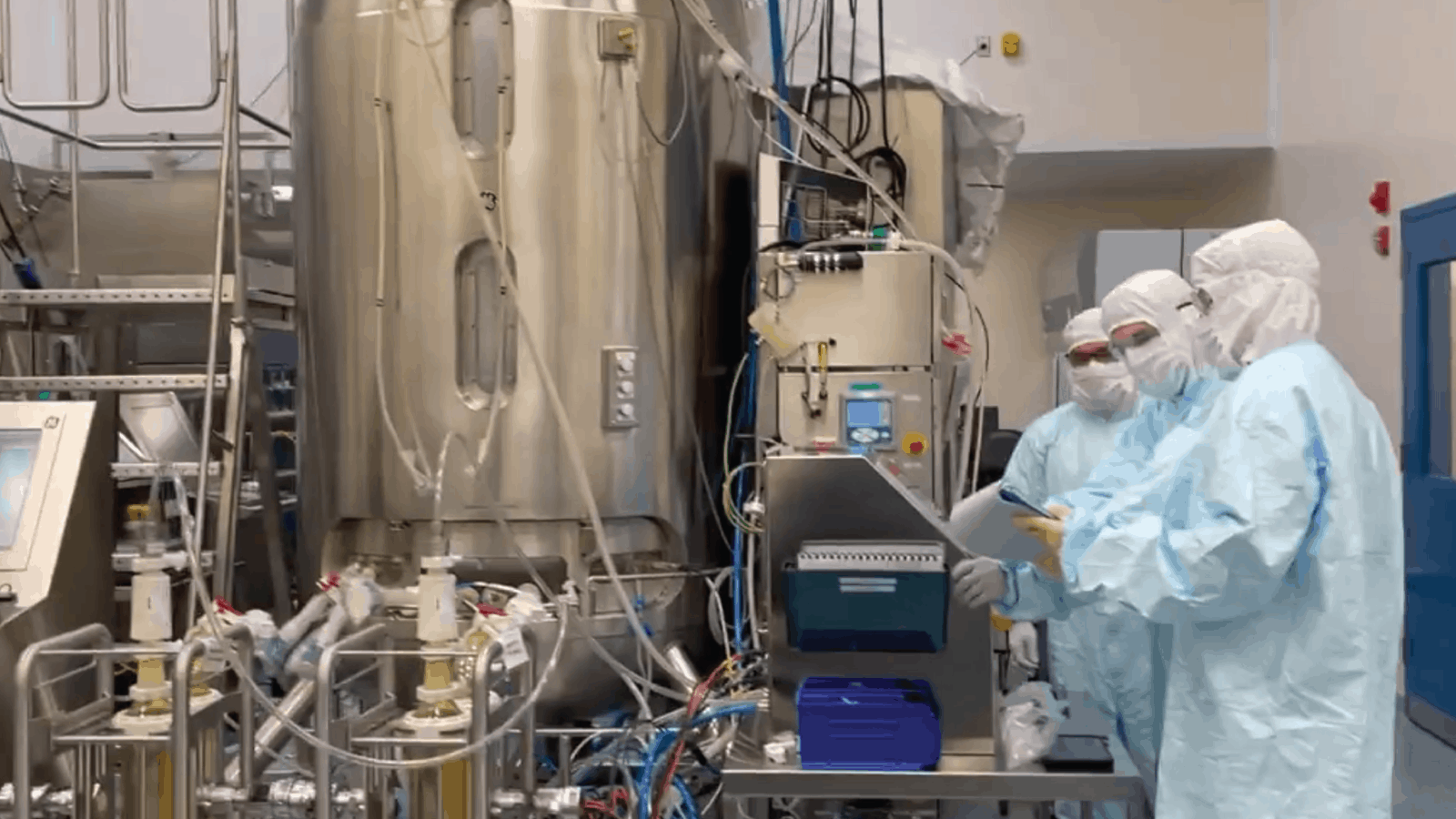
You may not know it, but one company has the capacity to manufacture bulk drug substance for more than a billion doses of COVID-19 vaccines annually: Emergent BioSolutions, a global supplier for the Johnson & Johnson vaccine and U.S. supplier for the AstraZeneca vaccine. Emergent Executive Vice President of Manufacturing and Technical Operations Sean Kirk spoke with us recently to explain what goes into the heroic production of all these doses—in other words, what it takes to help defeat COVID-19.
How the vaccine works: The complexity begins with the vaccines themselves, which are amazing feats of bioengineering. The two vaccines have broadly similar structures, though they are made by separate, quarantined production lines in the Emergent facility. (As Kirk says, you can’t even take a wrench from one production suite to the other.)
You can think of this type of vaccine as a sort of beneficial Trojan Horse:
- Particles of a virus called an adenovirus, which usually causes cold and flu-like symptoms, are engineered to hold the DNA of SARS-CoV-2 (the official name of the coronavirus)—and to not be infectious themselves.
- Those adenovirus particles enter your cells and program them to produce a component of SARS-CoV-2 called a spike protein.
- That process provokes an immune response, teaching your system how to defeat the real COVID-19.
So how do you make it? As you might guess, making such a precise vaccine is itself a complicated and delicate process.
- You need to make a lot of modified adenovirus particles very fast, while ensuring they aren’t infectious and can deliver their payload of SARS-CoV-2 DNA.
- To cut a long story very short, the production process involves “infecting living cells [with the modified adenovirus] and turning them into virus factories,” as science writer Derek Lowe says.
Where Emergent comes in: Emergent handles the manufacturing process, which results in something called “bulk drug substance,” Kirk explains.
- “Our facility produces the high concentration active pharmaceutical ingredients, the viral vectors themselves,” he says. “Then we freeze them down and ship them out to what’s called a fill/finish facility, which dilutes the concentrate and fills vials or syringes with it.”
The numbers: That concentrate will eventually become part of the 100 million Johnson & Johnson doses and 300 million AstraZeneca doses purchased by the U.S. government.
What it takes: Kirk gave us a glimpse of just how much effort went into getting ready for a new vaccine.
- 6 or 7 months: That’s all Emergent had, for a process that normally takes years. Consider how much goes into it, Kirk says: ordering equipment, getting that equipment to work correctly and comply with regulations, “working out the kinks from the complex biological manufacturing process”—and then scaling it up and optimizing it to make large quantities of vaccines as quickly and safely as possible.
- 800 new jobs: Emergent had to increase hiring, adding approximately 800 new jobs in 2020, many of which were dedicated to COVID-19 response across three Maryland sites.
- Group effort: Emergent works incredibly closely with Johnson & Johnson and AstraZeneca, along with the U.S. government and the company’s own suppliers. “We have leveraged U.S. government rated orders to get access to critical raw materials and equipment. We’ve depended upon certain suppliers, who were likewise rallying to the cause, to really step up and ramp up their overall capability and capacity,” says Kirk.
Why can’t you go faster? Kirk says he gets this question all the time and wants to impress upon readers that these are very complex biologic manufacturing processes.
- “They are highly regulated, highly technical and have to be highly reproducible,” he continued. “We are growing living cells and then we are infecting them with these viral vectors.”
- Furthermore, everything that Emergent produces must have the same characteristics of the product used in the clinical trials—“that’s the essence of biologic vaccine development,” Kirk says. “That’s the only way you can ensure safety and efficacy.”
The last word: Kirk tells us what he tells his employees: “It’s unbelievably difficult, more difficult than anything I’ve done in my entire career. But I can’t think of a more awesome opportunity to leave an indelible mark on the course of human history. We are going to help return a degree of normalcy to society. We’re going to help reunite families, open up economies and put a smile on children’s faces when they go back to school. And that’s an honorable and amazing thing.”
This article is the first in an exclusive four-part series on Emergent’s accelerated production efforts.
Tax Policy Makes Innovation Possible for Big Ass Fans

For Big Ass Fans, a Kentucky-based company that manufactures fans, evaporative coolers and controls for industrial, agricultural, commercial and residential use, the eye-catching name isn’t the only thing that makes them distinctive. The company is also a leader in research and development, crediting U.S. tax policy with supporting its innovations and the jobs they create.
Investing in innovation: BAF has spent millions of dollars in R&D, even building an R&D lab on its global headquarters campus in 2008. Most recently, it pioneered new ways of disinfecting air to keep manufacturing employees healthy during the COVID-19 pandemic. And when Congress approved tax reform in 2017—including a lower corporate tax rate—the company got additional fuel for its efforts.
- “The more incentives that are there for us to create and for our customers to purchase, the more we can deliver for everyone,” said BAF Government and Public Relations Director Alex Risen.
Risen cautions, however, that a higher corporate tax rate could impact the company’s ability to grow. Meanwhile, a prospective tax change on R&D spending could stymie innovation by requiring the amortization of expenses (as opposed to current tax policy, which allows expenses to be fully deducted in the same year).
- “We’re always going to innovate. That’s in our DNA. But if our customers have higher corporate tax rates, that can take money out of our pockets and theirs,” said Risen. “If this new R&D tax policy detracts from a company’s ability to push and pioneer…then we’re all at risk of losing out on expedited innovation.”
Creating American jobs: BAF isn’t just using its revenues to invest in innovation; it’s also working to bring jobs and supply chains into the United States. In addition to its headquarters in Lexington, Kentucky, the company has offices in Canada, Australia and Singapore. Up until recently, it also had a manufacturing facility in Malaysia in addition to a sales office there—but BAF is in the process of moving those production jobs to the United States.
- “It doesn’t just mean new jobs at BAF; it brings more business to American vendors and suppliers,” said Risen. “It allows them to continue trying to grow even during a downturn and uncertain times.”
Bolstering supply chains: In addition to job creation, strengthening the supply chain was another top priority for BAF.
- “We were already working on moving those operations before the pandemic hit, but the pandemic is a reminder that you want to have that supply chain close,” said Risen. “We’ve been fortunate that we haven’t had to slow production down, because the majority of our product is here in our backyard. That speaks to where we want to be as a company that is internationally headquartered in the U.S. but serves 175 countries. We want to do our part in order to make high-end machinery a U.S. export.”
NAM support: To support companies like BAF and its customers, the NAM is leading the effort to ensure that the tax code keeps encouraging innovation. Recently, a bipartisan group of U.S. policymakers introduced legislation that would allow manufacturers to continue to deduct their R&D expenses immediately—a move that the NAM advocated for. The NAM is also working to strengthen U.S. supply chains, releasing an agenda for such actions last year.
The bottom line: “A high tide floats all boats,” said Risen. “We need to continue to innovate and deliver for companies in America—and we need to help Americans push the envelope, innovate and deliver for all of us.”
So You Want to Run a Vaccination Site

Manufacturers across the country are doing their part for the pandemic response—whether that means developing vaccines, producing vials and containers or creating personal protective equipment for frontline responders. They are also increasing the capacity and efficiency of vaccination operations by embedding their manufacturing methods and technologies—as Honeywell and several partner organizations did recently in North Carolina. Now, the group has published a guide to help others do the same.
What they did: Honeywell, Atrium Health, Tepper Sports & Entertainment and Charlotte Motor Speedway formed a unique public–private initiative with a bold goal of distributing 1 million doses of the vaccine by July 4. With support from the state of North Carolina and Gov. Roy Cooper, the North Carolina Department of Health and Human Services and local governments, these organizations worked together to plan and execute efficient, safe and equitable mass vaccination events at Bank of America Stadium and Charlotte Motor Speedway in January and February.
- “These highly efficient mass events safely vaccinated a diverse group of more than 36,000 people with scalability at a rate of nearly 1,500 vaccinations per hour with average wait times of less than 30 minutes,” according to the guide. “These successes offer several best practices for locations around the world working to get ‘shots in arms’ quickly, efficiently and safely.”
Planning and structure: The guide encourages planners to offer doses by appointment only, to schedule the first and second doses concurrently and to ensure that the venue will have enough doses to serve all its guests without any waste. Meanwhile, it advises that a “task force” staff model be put in place with cross-functional teams and a clear decision-making structure.
Site selection: Planners should consider venues like stadiums, arenas, racetracks and convention centers as mass vaccination sites. But they should also consider whether these venues have:
- Sufficient space for social distancing;
- Free and available parking capacity if necessary; and
- Convenient access to public transportation.
Equity in distribution: Would-be vaccinators should take special account of underserved communities and populations, says the guide. Organizations seeking to create a mass vaccination site should engage in outreach, promote access and work to reduce vaccine hesitancy. That might require:
- Developing early partnerships with diverse faith-based, health care, business, educational, news and entertainment organizations;
- Working with the local government to create free transportation options; and
- Connecting with social influencers and community members who can help reduce vaccine hesitancy in targeted areas.
Process: This how-to guide lays out the processes an organization should be aware of and plan for—from pre-event scheduling to on-site check-in, screening, vaccination and observation. The organization should also plan to do post-event data entry, which ensures both their team and local governments can document doses correctly.
Why it matters: “Like any other successful endeavor, mass and community vaccination events require deep planning, strong leadership, committed partnerships and an army of support,” the guide says. “Missing even one of these critical elements can severely limit the effectiveness of an event, ultimately slowing down a community’s recovery… We hope these learnings will be helpful to government leaders who are building a strategy to get their community vaccinated.”
The last word: As NAM Vice President of Brand Strategy Chrys Kefalas said, “Manufacturers like Honeywell and their partners in health care and government are leading us toward the end of the pandemic. It’s important that all of us play our parts to help them, as the NAM and The Manufacturing Institute’s ‘This Is Our Shot’ project emphasizes. Our industry has been protecting Americans from COVID-19 for a year now, and our job isn’t over yet.”
You can download the full guide here.
NAM Board Reelects Lamach and Fitterling

The NAM Board of Directors has reelected Trane Technologies Chairman and CEO Mike Lamach as its chairman and Dow Chairman and CEO Jim Fitterling as vice chair.
Lamach and Fitterling provided stalwart leadership during an extraordinarily difficult year. Under their guidance, the NAM achieved notable successes, ensuring that policymakers accounted for the industry’s needs and helping to make the production of masks, vaccines and other vital supplies possible.
And that’s not even the half of it. Here are some of the highlights from the NAM’s past year:
- COVID-19 response: Our “American Renewal Action Plan” shaped legislation and administrative action to get manufacturers the support they needed. The NAM team’s advocacy secured more than six dozen policy accomplishments.
- PPE production: Our Creators Respond initiative helped send millions of pieces of personal protective equipment and other medical supplies to hospitals and health facilities—and after the election, the NAM worked with the Biden transition team to share insights on PPE production and distribution.
- Workforce development: The Manufacturing Institute’s initiatives, including Heroes MAKE America, the STEP Women’s Initiative and the FAME apprenticeship program, strengthened manufacturing’s workforce pipeline and helped close the skills gap.
- Legal victories: The NAM led the business community in court on issues like protecting vital immigration and standing up against regulatory overreach.
- Fight for opportunity: Through our Pledge for Action, the NAM has committed our sector to taking 50,000 tangible actions to increase equity and parity for underrepresented communities and creating 300,000 pathways to job opportunities for Black people and all people of color.
A look ahead: With Lamach and Fitterling at the helm and an exceptional team in place, the NAM is poised to expand on its successes over the past year and continue to strengthen manufacturing across the country. Already, the NAM is working closely with the new administration and Congress to make sure manufacturers’ voices are heard. To learn more about the breadth of the NAM’s policy agenda, read its newly updated blueprint “Competing to Win.”
Small and medium-sized manufacturers: Meanwhile, the current chair of the NAM’s small and medium-sized manufacturers’ group, BTE Technologies President Chuck Wetherington, will also serve another two years in his position. Ketchie President and Owner Courtney Ketchie Silver will replace retiring Protolabs President and CEO Vicki Holt as vice chair.
The last word: “Today more than ever, manufacturers are the arsenal of democracy. In our nation’s time of need, manufacturers have stepped up and manned the front lines to provide essential goods for the American people. With Mike and Jim’s sound guidance and experience, the NAM will continue to be a leading voice for the business community during these unprecedented times,” said NAM President and CEO Jay Timmons.
“Our board leaders will also help our industry lead America’s recovery and renewal—helping to strengthen and unify our nation during extraordinary times. And above all, we will advance the values that make America exceptional: free enterprise, competitiveness, individual liberty and equal opportunity.”
What Manufacturers Should Know About Vaccines
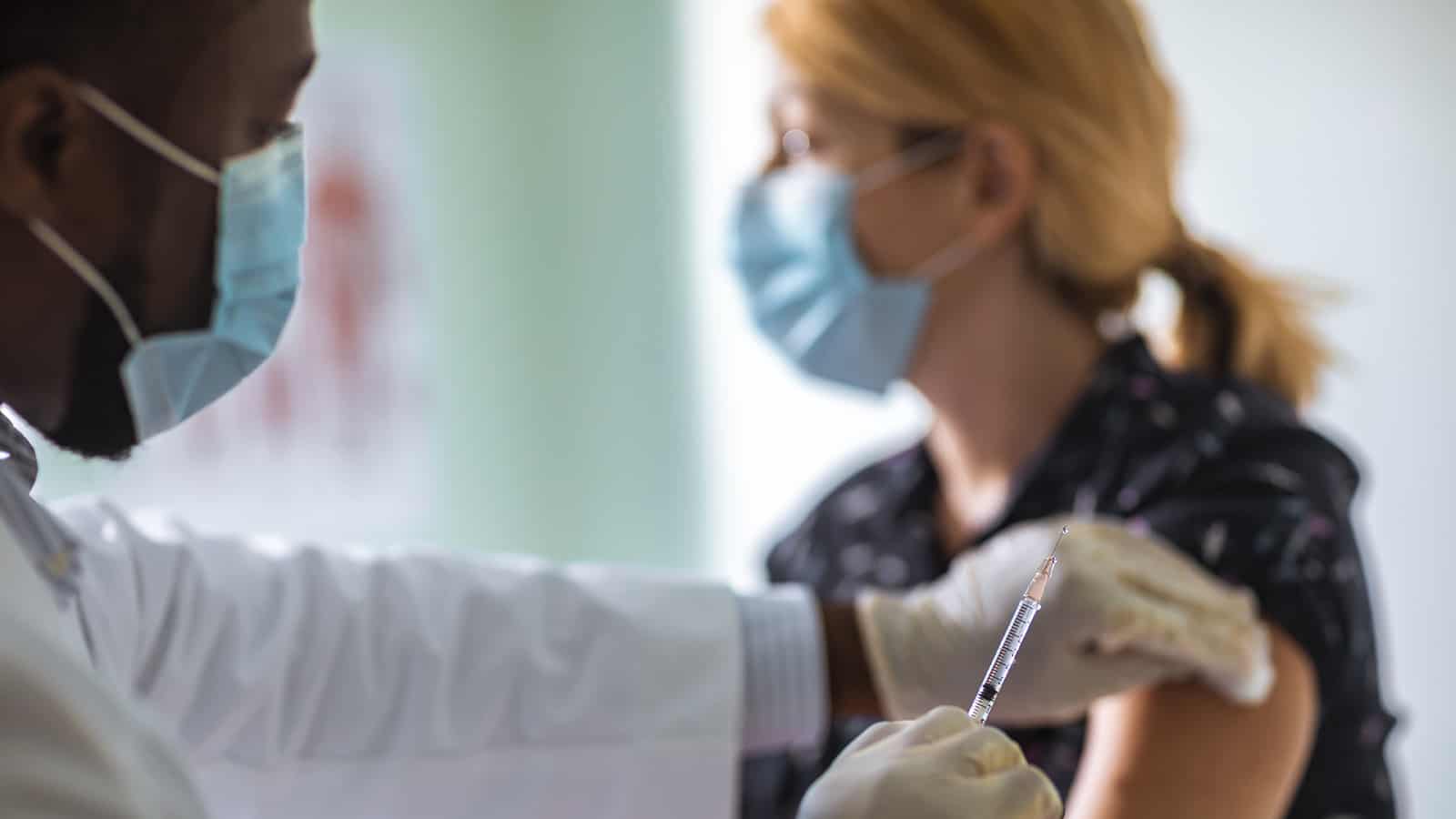
As we wait to get our shots, many people still have questions. Does it matter which vaccine I get? What safety precautions should I continue to take? We talked to highly cited infectious disease expert Dr. Aaron Richterman of Penn Medicine to get some answers to these very real concerns.
Which vaccine? As Richterman tells us, the priority for vaccines is preventing bad outcomes—death and severe illness. And the good news? “The really, really good news is that all of these vaccines that have been tested so far—all of them—prevent severe outcomes.” That list includes vaccines made by Pfizer, Moderna, Johnson & Johnson, AstraZeneca and Novavax. (Only Pfizer and Moderna are approved in the U.S. so far, while the J&J vaccine is expected to be approved soon.)
- And the clincher? “When you put all the trials together [including the Russian vaccine trial], there are somewhere around 80,000 to 90,000 people total who have received the vaccine, and none of them have required hospitalization—and none have died,” says Richterman.
Let’s talk specifics: What about the different numbers we’ve been seeing? As Richterman points out, we’re all used to seeing different headline numbers—95% effective, or 72%, and so forth. But what does that really mean?
- What those numbers represent is the “reduction in any symptomatic illness at all,” he explains. But the main thing we should be concerned about is the prevention of severe illness or death—which all the vaccines do extremely well.
When should I get it? As soon as possible, says Richterman. “At this point in time, and probably for the next four to six months in this country, the benefit of getting the first vaccine available to you is going to outweigh any potential benefit from waiting for the next one.” If you are offered an FDA-approved vaccine now, he says: “take it.”
What you should know: Here are some key facts to keep in mind as you read media coverage of vaccinations, says Richterman.
- Quality: “The quality of evidence underpinning the data for these vaccines […] is grade A plus, top of the line.”
- Safety: “These are extremely safe vaccines, among the safest out there. Some people will have temporary side effects, but they are very safe.”
- Prevention of severe outcomes: These vaccines prevent severe outcomes to a “tremendous” degree, Richterman stresses. “If these vaccines can take COVID-19 down to something asymptomatic, or something more like a cold, that’s a big win.”
And lastly, do you have to wear masks and socially distance after being vaccinated? “Especially right now, when there’s a lot of community transmission,” Richterman says, “and we’re still learning about new variants, it’s a good idea to keep things as safe as possible.” However, “people should be informed that the vaccine reduces their risk and the risk for those around them.”
Further reading/viewing: Check out the NAM’s project to increase vaccine acceptance, This Is Our Shot, as well as its continued efforts to promote the use of face coverings.
Caterpillar Powers COVID-19 Relief Efforts

Manufacturers have pitched in remarkably to help us all through COVID-19—by making things you might expect to be essential (masks, gowns) and things you might not (wire racks, foam). One major manufacturer, Caterpillar, has contributed to the relief efforts in a wide variety of ways across the country and around the world. Here are some of its contributions.
Heating hospital tents: Caterpillar generators made a key appearance in Atlanta during the worst of the 2020 spring surge. Local hospitals set up testing sites outdoors so they could admit only confirmed COVID-19 cases, thus keeping noninfected patients safer from exposure. But to do that, they needed power.
Cat® Dealer Yancey Power Systems provided exactly the power they needed, after thoroughly evaluating the sites, making a detailed plan for servicing them safely and creating an easy set-up process.
Helping to make face shields: A Peoria manufacturer of face shields was missing a critical component—and local hospitals desperately needed those shields. The manufacturer called a Caterpillar distribution center at about 9:00 one morning and had what it needed just an hour later.
Donating materials: And speaking of face shields, a Caterpillar facility in Brazil donated production materials for face shields to local manufacturers, so that doctors in their hard-hit region would be better protected.
Feeding people: The Caterpillar team in Seguin, Texas, hosted two huge food distribution events in 2020. The event in May provided more than 700 families with enough food for two weeks, while in December, the Caterpillar Seguin team and the local food bank provided food to 955 families from 10 different counties.
Powering tugboats: Remember the rousing scene in March when the USNS Comfort, a Navy hospital ship, sailed into New York Harbor? You may not recall the two tugboats that helped it dock, but they were essential. And inside those tugboats were two Caterpillar engines, which ensured the boats could serve their city when it needed them.
Providing support: The Caterpillar Foundation also committed $10 million to support global and local COVID-19 response activities. Together with the incredible outpouring of support from employees and retirees through the Foundation’s special 2:1 match, these contributions have helped keep communities safe and strong.
The last word: “Our employees, dealers and customers around the globe are doing what they can to help fight the spread of COVID-19 and ensure essential work continues,” says Kathryn Karol, Caterpillar vice president of global government and corporate affairs. “They truly embody our values in action, finding ways to help each other and their communities during these difficult days.”
